
What is the Lewis structure of \ce{H2CO3}? Quizlet
A step-by-step explanation of how to draw the H2CO3 Lewis Dot Structure (Carbonic Acid). For the H2CO3 structure use the periodic table to find the total number of valence electrons.

H2CO3 Lewis Structure (Carbonic Acid) YouTube
Lewis Structure of Carbonic Acid (H2CO3) The formula of carbonic acid is H2CO3. It has two H atoms, one C atom, and three O atoms. To understand the molecular formula of H2CO3, we have to observe the electronic configuration of the participating atoms and how many atoms they have in the outer shell.

Carbonic acid Molecular Geometry Hybridization Molecular Weight
A step-by-step explanation of how to draw the HCO3- Lewis Dot Structure (Hydrogen Carbonate or Bicarbonate Ion).For the HCO3- structure use the periodic tabl.
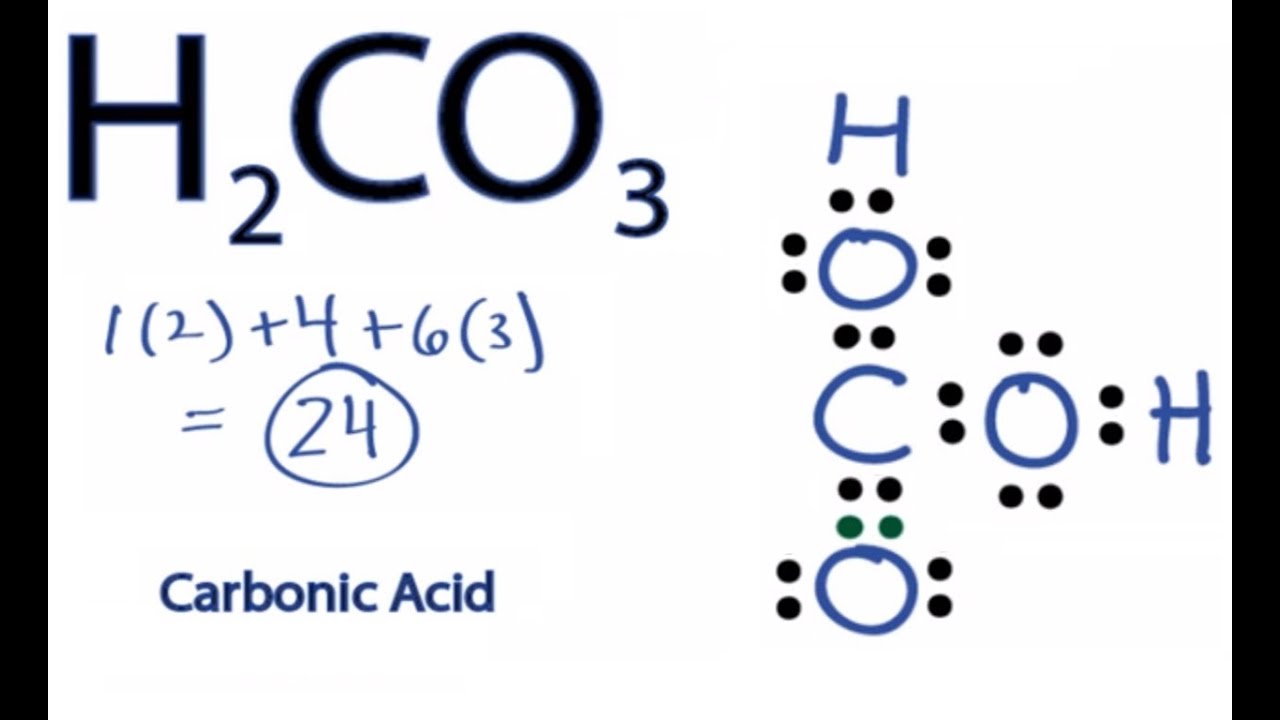
H2CO3 Lewis Structure How to Draw the Lewis Structure for Carbonic
Structural Formula. H 2 CO 3. carbonic acid

H2co3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram
A step-by-step explanation of how to draw the H2CO3 Lewis Structure (Carbonic Acid). When we have an H (or H2) in front of a polyatomic molecule (like CO3.
[Solved] There are three possible resonance structures for carbonic
For the H 2 CO 3 Lewis structure (Carbonic Acid) make sure you put the Hydrogen atoms on the outside of the oxygen atoms. With H 2 CO 3, Carbon (C) is the least electronegative and goes in the center of the structure. There are a total of 24 valence electrons in H 2 CO 3. H2CO3 Lewis Structure: How to Draw the Lewis Structure for Carbonic Acid
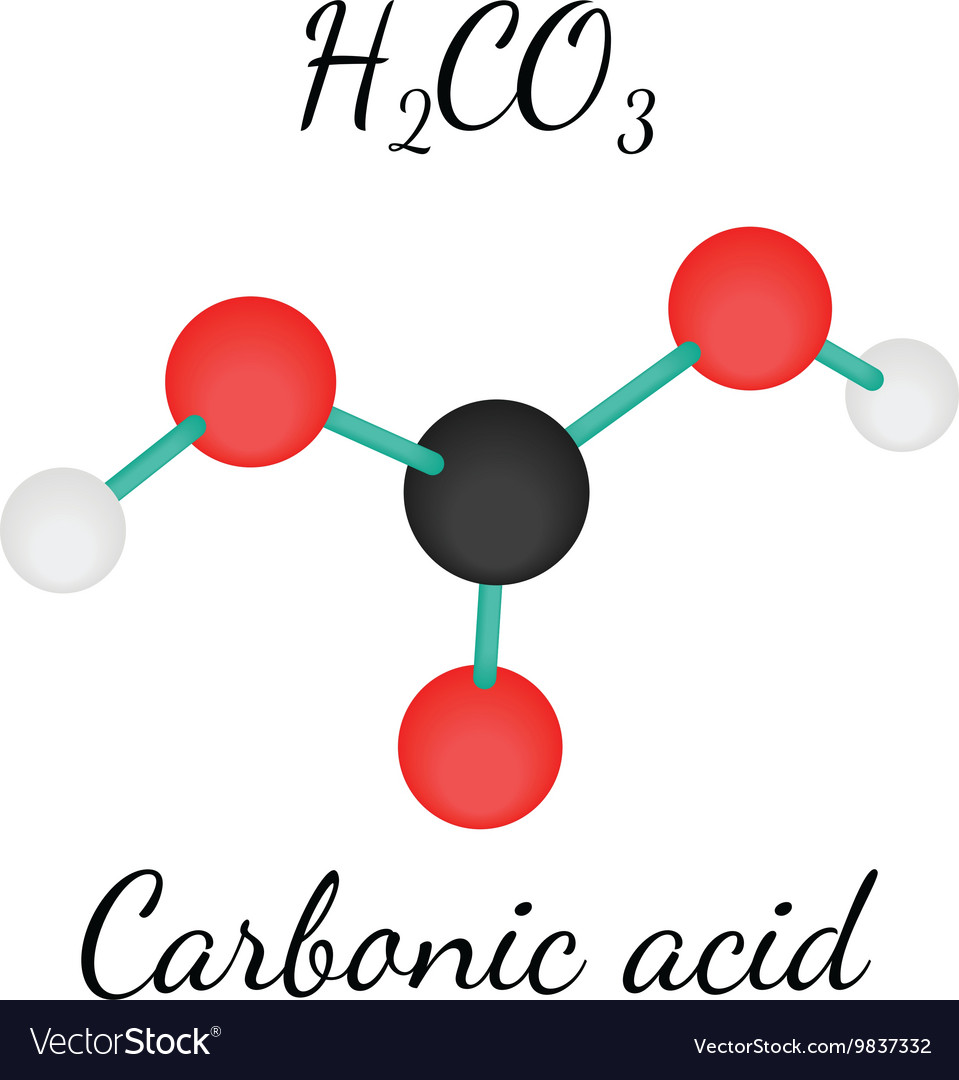
H2CO3 Carbonic acid molecule Royalty Free Vector Image
Carbonic acid is a carbon oxoacid and a chalcocarbonic acid. It has a role as a mouse metabolite. It is a conjugate acid of a hydrogencarbonate. Carbonic acid is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). Carbonic acid (H2C03). The hypothetical acid of carbon dioxide and water.
[Solved] What will be the charge of the ion formed from each of these
H2CO3 lewis structure has a Carbon atom (C) at the center which is surrounded by one Oxygen atom (O) and two O-H groups. There is 1 double bond between the Carbon atom (C) & Oxygen atom (O) and the rest other atoms have a single bond. There are 2 lone pairs on all three Oxygen atoms (O).

lewis dot structure of h2co3 is Chemistry 16239199
Like ozone, the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure. Unlike O 3, though, the actual structure of CO 3 2 − is an average of three resonance structures. 1. Because carbon is the least electronegative element, we place it in the central position:

H2CO3 Lewis dot structure YouTube
Lewis structure of H2CO3 (Carbonic acid) contains one double bond between the Carbon atom (C) & one Oxygen atom (O) and the rest other atoms are single bonded with each other. The Carbon atom (C) is at the center and it is surrounded by one Oxygen atom (O) and two O-H bonds. All the three Oxygen atoms have 2 lone pairs.

[Chemistry] Lewis Structure for H2CO3 Does it matter how it looks
H2CO3 is one of the most known chemicals and is a chemical formula for Carbonic acid. In today's video, we help you determine its Lewis Structure by followin.

H2co3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram
Page Contents show How to draw lewis structure of H2CO3? The Lewis structure of carbonic acid (H2CO3) consists of a carbon (C) atom at the centre. It is double-covalently bonded to an oxygen (O) atom on one side and single-covalently bonded to two hydroxyl (OH) functional groups on the other two sides.
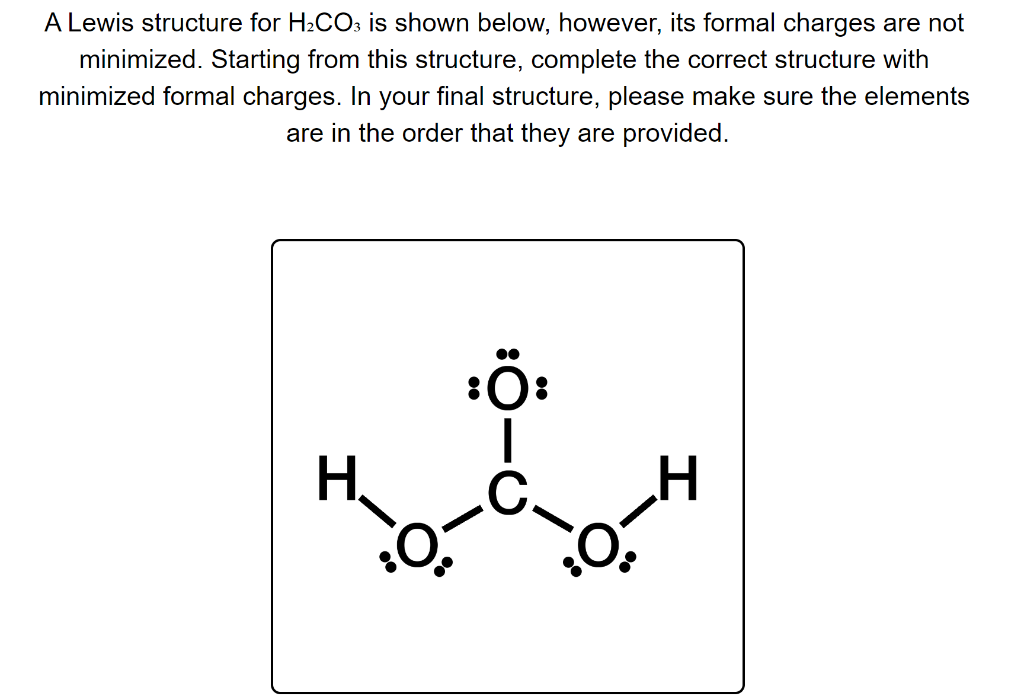
Solved A Lewis structure for H2CO3 is shown below, however,
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

What is the correct Lewis structure for carbonate acid , H2CO3
By using the following steps, you can easily draw the lewis structure of H 2 CO 3. Step #1: draw skeleton. Step #2: show chemical bond. Step #3: mark lone pairs. Step #4: complete octet on central atom. Step #5: calculate formal charge and check stability. Let's one by one discuss each step in detail.

H2CO3 Lewis Structure, Molecular Geometry, Hybridization, and MO
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.
Lewis Dot Structure For H2co
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of.